Building on Two Decades of Successful Transcatheter Closure Device Experience
The Encore Patent Foramen Ovale (PFO) Closure Device provides a safe and effective option for transcatheter closure of the patent foramen ovale defect. Once delivered to the location of the defect via the delivery catheter, the device quickly closes the unwanted communication between the right and left atria. This prevents clots from traveling through the PFO and potentially eliminates them as a source of embolic stroke.
Encore Medical was formed in October of 2020, but that is not the beginning of our story. The company and its products are based on 20+ years of transcatheter closure device experience outside the United States. Encore Medical’s devices are based on a patented technology platform which has successfully implanted more than 25,000 heart defect closure devices over the past two decades. This knowledge and experience have allowed Encore Medical to create world-class closure devices for patients in need.
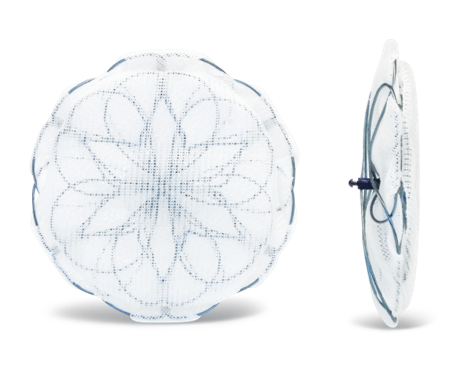
Encore PFO Closure Device
The Encore PFO Closure Device represents the culmination of two decades of technological advances in transcatheter defect closure devices. The incorporation of these advances yielded a device with the following benefits:
- Ease of implantability
- Full retrievability
- Effective closure of the defect
- Minimal blood flow disturbance after implant
- Minimal force exerted on atrial tissues
- Conformance to the anatomy in multiple dimensions
- Does not limit future access to the left atrium via the foramen ovale
Encore ASD Closure Device
The Encore ASD Closure Device is based on similar technology to that used in the Encore PFO Closure Device. The device offers a safe and reliable method of closing an atrial septal defect (hole between the atria) in both children and adults. The Encore ASD Closure Device is available in multiple sizes to accommodate defects in most patients. The device offers the following key benefits:
- Ease of implantability
- Full retrievability
- Effective closure of the defect
- Low metallic surface area
- Self-centering design
- Does not limit future access to the left atrium via the foramen ovale

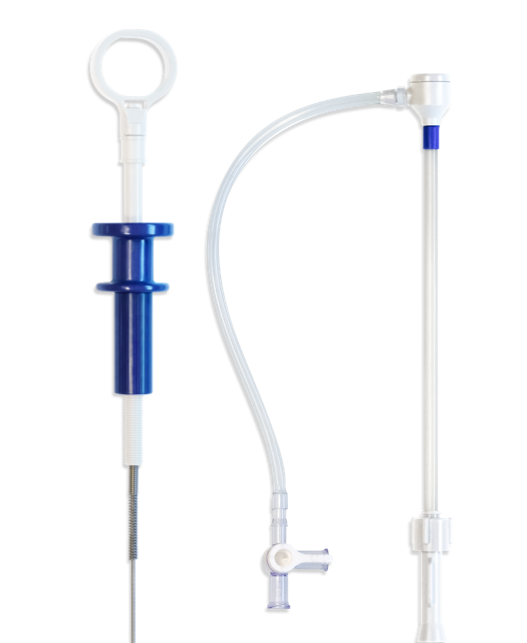
Encore Delivery System
The Encore Delivery System provides physicians with an intuitive catheter system to implant any Encore Closure Device (PFO or ASD). The system contains the following key features:
- Sizes to fit the selected device: 9, 10, 11 and 12 Fr.
- Delivery sheaths have “J” shape to provide optimal implant angle
- Self-sealing hemostasis introducer
- Positive locking device release system
- Radio opaque tips
Randomized Trial of the Encore PFO Closure Device –
The PerFOrm Trial
Randomized Trial of the Encore PFO Closure Device – The PerFOrm Trial – will compare the Encore PFO Closure Device to currently FDA approved devices. The data provided by this trial is intended to support the approval of the Encore PFO Closure Device in the United States. The PerFOrm Trial will also provide the data needed to make direct comparisons between the Encore PFO Closure Device and other devices.
Who Qualifies
Patients who have had a cryptogenic stroke and have a PFO may qualify for the trial.
Get Involved
Encore is currently recruiting qualified investigators. Interested physicians should contact Encore Medical.
News and Information
Retrospective review of thienopyridine therapy in migraineurs with patent foramen ovale
We retrospectively reviewed our clinical experience using off-label thienopyridine agents in patients with migraine headache…
Ticagrelor for Refractory Migraine/Patent Foramen Ovale (TRACTOR)
After finding that the thienopyridines clopidogrel and prasugrel reduced migraine headache (MHA) symptoms in some…
The Multiple Clinical Manifestations of Patent Foramen Ovale
A patent foramen ovale (PFO) is a congenital remnant of the fetal circulation that persists…
Patient Stories
These patient stories from the news highlight the real-world impact of stroke in people diagnosed with a patent foramen ovale (PFO). The individuals featured have not been treated with or used our PFO closure device; their experiences are shared to provide broader context around this condition.
ABC News
Donald Glover's stroke and 'hole in his heart': What are the early warning signs?
Watch from beginning of video to :50, then 1:40 to end.
Good Morning America
38-year-old woman suffers stroke caught on camera; later diagnosed with PFO
Watch from beginning of video to 3:15 timestamp.